Hydrophobic or hydrophilic
It is not an exaggeration to consider fog formation as a worldwide problem that must be solved. The NASA astronauts came across this issue years ago in their firsts space missions but any common person can deal with this problem while driving his car on a rainy day.
A common problem
The physical explanation for this phenomenon is based on the thermodynamics laws and the light-matter interaction. When an amount of water in vapour state contacts with a cold and less energetic surface, it starts transferring energy to it. Then the water condensates and vapour becomes tiny water droplets. The presence of irregularly shaped water pieces will enhance the medium dispersion towards the light, as dispersion is the opposite of transparency it remains clear that the fog formation will imply a loss of visibility through any surface.
Hydrophobic and hydrophilic surfaces
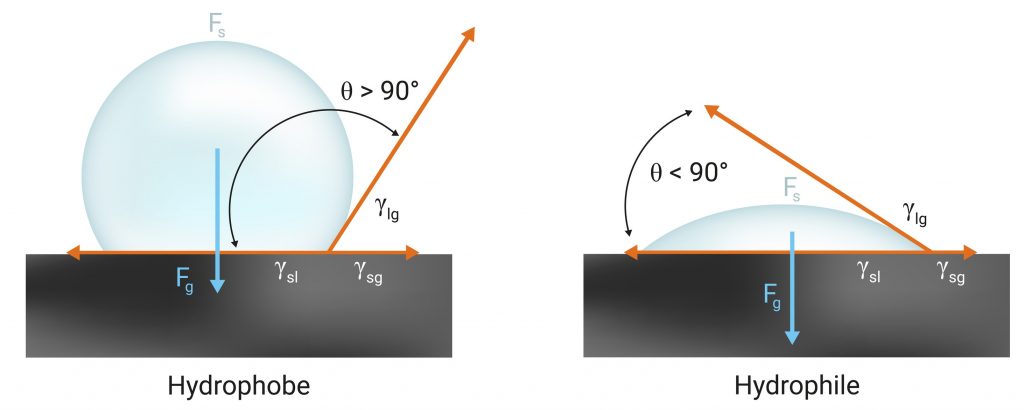
A good way to parameterize the behaviour of a surface with water is the static contact angle θ. As it is shown in the picture, it determines how a water droplet interacts with a static horizontal surface under gravitational effects. The contact angle can be characterized mathematically by the differences between the superficial tensions involved: air-water, air-surface and water-surface.
A surface is hydrophobic if the contact angle with water is greater than 90º. Intuitively, as θ angle increases, there is less contact surface between the spherical droplet and the surface, thus making the droplet slip on the surface without adhering. The tulip poplar leaf is a natural resource that offers great hydrophobic properties. These properties can also be found in industrial materials as many plastics and in highly developed surfaces based on silicon oxides.
Hydrophilicity is identified by a static contact angle with water smaller than 90º. A hydrophilic surface will tend to absorb or attract water, in other words, it is more wettable. Nature also offers substances that highly interact with water, for example, we could mention soluble composites such as sugar, salt or starch.
The property that enhances their hydrophilicity is based on the electrical polarity of water molecules. Some chemical composites as hydroxides attract water molecules creating the so-called hydrogen bondings. This chemical property is the motivation to create thin films where there is an abundance of hydroxides to provide a material with hydrophilic behaviour.

Which properties are needed to create an anti-fog?
It seems reasonable to think that a hydrophobic surface will prevent fog formation because water droplets will have more difficulties to stay on it and thus, there won’t be irregularities that disperse light. That simple reasoning leads to a vast technical field that aims to obtain anti-fog results by applying hydrophobic coatings.
An example of a hydrophobic solution uses nanostructures made of periodical nanopillars. These surfaces are transparent and avoid fog formation because water droplets do not stay on them.
However, it’s even greater the interest in the anti-fog coatings based on enhancing hydrophilic properties of the surfaces, most of the products that are currently used are based on this idea. The argument to this is not as straightforward as the previous one. A hydrophilic surface, in general, will attract water.
Paradoxically, this water abundance will tend to organize all the droplets in a homogeneous thin film which won’t disperse light, creating a transparent layer without disrupting the general transparency of the set. In these kinds of solutions, it’s also needed a procedure to eliminate the excesses of water.
The efficiency of a hydrophilic surface can be improved by wetting it with a surfactant. A surfactant is understood as a compound that reduces water-surface tension and thus the static contact angle θ is decreased.
Hydrophobic anti-fog: From theory to real solutions
PhotonExport offers an anti-fog product that can provide permanent thin film coatings by using physical vapour deposition techniques such as resistance evaporation or electron beam evaporation. The product is made of polarized compounds that increase the hydrophilicity of the surface where it is applied.
The variety of investigation paths is nothing that good news to the lively scientific and technological sector of the anti-fog solutions based on coatings. A widespread problem as the fog formation must have a vast range of possible solutions that can be applied in different applications as an anti-fog applied to ophthalmic lenses or an anti-fog applied to bath mirrors.
Click here to view our hydrophobic coating materials.
